Atoms and Elements Of course. Here is a comprehensive overview of atoms and elements, from the basic definitions to more advanced concepts.
The Core Definitions
- An Atom is the smallest unit of ordinary matter that forms a chemical element. It is the basic building block of chemistry. Every solid, liquid, gas, and plasma is made up of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers (a ten-billionth of a meter) across.
- An Element is a pure substance consisting only of atoms with the same number of protons in their atomic nuclei. This number is called the atomic number. We cannot break down an element into simpler substances by chemical means.
- Example: Every single atom of gold (element symbol Au) has exactly 79 protons in its nucleus. If an atom has 80 protons, it is no longer gold; it’s mercury (Hg).
- Analogy: Think of elements as different types of LEGO bricks (red bricks, blue bricks, long bricks, wheel bricks). Individual atoms are the single, physical LEGO bricks themselves.
The Structure of an Atom
- The Nucleus: The dense, central core of the atom, containing protons and neutrons. It contains almost all of the atom’s mass but takes up virtually none of its space.
- The Electron Cloud: The area surrounding the nucleus where electrons exist. It’s mostly empty space but defines the volume (size) of the atom. Electrons move so fast they form a “cloud” of possible locations.
- Key Principle: A neutral atom has no overall electric charge. This means the number of protons equals the number of electrons.
Atomic Number and Mass Number
- Example: Carbon’s atomic number is 6. Every carbon atom has 6 protons.
- Mass Number (A): The total number of protons and neutrons in an atom’s nucleus (A = Z + N, where N is the number of neutrons).
- Example: A carbon atom with 6 protons and 6 neutrons has a mass number of 12.
Isotopes and Ions: Variations on an Atom
- Atoms of the same element can vary in their number of neutrons and electrons.
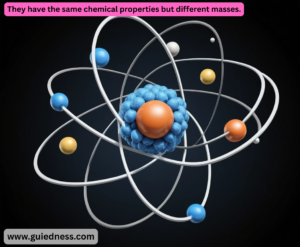
- Isotopes: Atoms of the same element (same atomic number) that have different numbers of neutrons (and therefore different mass numbers).
They have the same chemical properties but different masses.
- Example: Carbon-12 (6 protons, 6 neutrons) and Carbon-14 (6 protons, 8 neutrons) are both isotopes of carbon.
- Ions: Atoms (or groups of atoms) that have gained or lost electrons, giving them a net electric charge.
- Cation: A positively charged ion (formed by losing electrons).
- Example: A sodium atom (Na) loses one electron to become a sodium ion (Na⁺).
The Periodic Table of Elements
- The Periodic Table is a systematic chart of all known chemical elements, organized by their properties.
- Organization: Elements are listed in order of increasing atomic number (Z).
- Rows are called Periods: The period number indicates the highest energy level (shell) an electron occupies in its ground state.
- Columns are called Groups (or Families): Elements in the same group have the same number of valence electrons, which gives them very similar chemical properties.
- Example: Group 1: Alkali Metals (Li, Na, K, etc.) are all highly reactive metals.
- Example: Group 18: Noble Gases (He, Ne, Ar, etc.) are all very stable and unreactive.
Key Areas of the Table:
- Metals: On the left side and center (e.g., iron, copper, aluminum). They are typically shiny, malleable, ductile, and good conductors of heat and electricity.
- Nonmetals: On the right side (e.g., oxygen, carbon, nitrogen).
- Metalloids: Along the zig-zag line (e.g., silicon, boron). They have properties intermediate between metals and nonmetals.
Beyond the Basics: Advanced Atomic Structure
The Quantum Model
- The simple “solar system” model of the atom (nucleus with electrons orbiting in neat circles) is outdated. The modern understanding is based on quantum mechanics.
- Electron Orbitals: Electrons don’t orbit in fixed paths. Instead, they exist in orbitals, which are three-dimensional regions around the nucleus where there is a high probability (over 95%) of finding an electron.
- Energy Levels (Shells): Orbitals are grouped into energy levels (shells) labeled n=1, 2, 3, etc. The higher the number, the higher the energy and the farther the electron is from the nucleus.
- Subshells (s, p, d, f): Each energy level contains subshells of different shapes:
s-orbital: Spherical
p-orbital: Dumbbell-shaped
d and f orbitals: More complex shapes
- This model explains the chemical behavior of elements, as it describes how atoms interact and bond based on their electron configurations.
Electron Configuration
- This is a notation that shows the distribution of electrons in an atom’s orbitals. It’s the reason the periodic table has its distinctive shape.
- Example: Sodium (Na, atomic number 11) has an electron configuration of 1s² 2s² 2p⁶ 3s¹.
- Valence Electrons: The electrons in the outermost shell (the 3s¹ electron in sodium). These are the electrons involved in chemical bonding. The number of valence electrons is the key to an element’s reactivity and is directly linked to its group number on the periodic table.
How Elements Form Everything: Chemical Bonding
- Atoms (except noble gases) are not stable on their own. They seek to gain, lose, or share electrons to achieve a stable electron configuration, typically with 8 valence electrons (the “Octet Rule”). This drive leads to bonding.
The Origin of Elements: Nucleosynthesis
- Elements weren’t just created at the same time. They were forged in the universe’s most violent events:
- The Big Bang: Created the lightest elements: Hydrogen, Helium, and trace amounts of Lithium.
- Stellar Nucleosynthesis (Fusion): Inside stars, smaller nuclei fuse together to create larger ones under immense heat and pressure. This forges elements up to Iron (Fe).
- Example: Hydrogen fuses into Helium, which fuses into Carbon and Oxygen, and so on.
- Supernova Nucleosynthesis: When massive stars die in supernova explosions, the incredible energy allows for the creation of elements heavier than Iron, like gold, silver, lead, and uranium. These explosions then scatter these elements across the cosmos, where they form new stars, planets, and eventually, us.
We are literally made of stardust.
Key Concepts to Remember
Atomic Mass vs. Atomic Weight:
- Atomic Mass: The mass of a specific isotope (e.g., the mass of Carbon-12 is 12 amu).
- Atomic Weight: The weighted average mass of all the naturally occurring isotopes of an element. This is the number you see on the periodic table (e.g., Carbon’s atomic weight is 12.01 amu).
- Mole: A fundamental chemistry concept. One mole of any substance contains exactly 6.022 × 10²³ particles (Avogadro’s number). This number allows chemists to count atoms and molecules by weighing them.
- States of Matter: Elements can exist in different states (solid, liquid, gas, plasma) based on temperature and pressure. These are physical changes that do not alter the identity of the element’s atoms.